PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Eucalyptus foliage diseases
Scion is the leading provider of forest-related knowledge in New Zealand
Formerly known as the Forest Research Institute, Scion has been a leader in research relating to forest health for over 50 years. The Rotorua-based Crown Research Institute continues to provide science that will protect all forests from damage caused by insect pests, pathogens and weeds. The information presented below arises from these research activities.
From Scion publication Forest Research Bulletin 220,
An Introduction to The Diseases of Forest and Amenity Trees in New Zealand.
G.S.Ridley and M.A. Dick 2001.
There are a great many fungi causing or associated with leaf spots on eucalypts. Those covered in this section are representative, and include the diseases which have caused the most significant damage to plantation species in New Zealand.
Leaf symptoms tend to progress from discoloration and death of small areas of tissue through to involvement of most, or all, of the leaf or needle area, followed by casting. The distribution of lesions is sometimes related to the structure of the leaf, spread being contained by the veins, but often they are scattered haphazardly and may spread and coalesce without restriction. Leaf spots are not only due to biotic attack but may also be the result of abiotic agencies such as chemicals or of physical injury. Chlorosis is often associated with mineral deficiencies, variegated patterns with viral diseases. Large-scale withering of the leaf is often referred to as blight.
Common name: Eucalypt leaf spots
Country of origin: Australia
Host(s):
Symptoms and disease development:
Symptoms of many of the leaf diseases are very similar, and only microscopic examination can distinguish between them. There are a few with very distinctive symptoms, e.g., the bright yellow and carmine colours caused by Phaeophleospora eucalypti, or the typical buckling of the leaves associated with Mycosphaerella cryptica. For the non-specialist a knowledge of the host range of the different diseases can be very helpful in determining which disease is present when attempting a diagnosis. Symptoms are most severe in areas favouring extended periods of leaf wetness. Such conditions can occur at high altitude where mist is prevalent; in gullies, depressions, and valleys protected from wind; and in areas of closely planted young trees. Species or provenances growing off-site are more prone to infection.
In general Mycosphaerella cryptica, M. nubilosa, and Phaeophleospora eucalypti cause spreading blotches. Aulographina eucalypti and Trimmatostroma bifarium and T. excentricum cause roughly circular, brown spots/ often with raised corky patches. Sonderhenia eucalyptorum and M. swartii form minute spots, seldom more than 3 mm in diameter, and Phaeothyriolum microthyrioides is visible as black fruit-bodies on the leaf surface, sometimes causing leaf discoloration. Pseudocercospora eucalyptorum causes angular brown lesions.
Aulographina eucalypti
Aulographina eucalypti has been found on the green leaves (Fig. 13) and twigs of a wide range of Eucalyptus spp. Leaf lesions are initially very small and appear corky due to the formation of brown callous cells. Lesions continue to grow over a period of time and may become large, roughly circular spots.

Fig, 13: Leafspots caused by Aulographina eucalypti on Eucalyptus regnans
A distinctive feature of this disease is that lesions often do not penetrate right through the leaf because of the formation of a meristem with cork-like cells in the healthy tissue beneath the infection. Initially, small, circular, shield-like fruit-bodies of the anamorph develop on the surface of the lesions, followed by black, elongated, frequently branched fruit-bodies of the teleomorph. Spores are wind-dispersed and are discharged during periods of high humidity. In trees that are largely healthy, leaves low in the crown that have been weakened by ageing become infected. Only mature leaves are susceptible to infection. Aulographina eucalypti grows slowly in young leaf tissue and symptoms of infection on current season leaves are not usually visible until autumn. This is in contrast to infection by Mycosphaerella spp. where the fungi rapidly invade newly formed leaves and leaf spots can be seen during the period of active growth.
Mycosphaerella cryptica
In the early stages of symptom development, lesions caused by Mycosphaerella cryptica are red-brown in colour and frequently have a prominent purple margin. As the fungus develops, the lesion changes in colour to pale grey and then dark grey (Fig. 14 and 15). Old infected leaves may be riddled with holes where necrotic tissue has dropped out (cf. shothole). Leaves are often badly distorted and those with extensive infection are readily abscissed. In association with this leaf attack, some host species are infected on the shoots and young twigs (Fig. 16). Cankers up to 25 mm long develop, the bark splits longitudinally, and gummosis may occur. Dieback follows when cankers girdle the twigs and shoots and as a result thin crowns and dead tops become very apparent. Symptoms of this disease can appear very similar to frost damage.
The conidiospores of Mycosphaerella cryptica are produced primarily on young lesions and are present mainly from December to March. Mature ascospores are present throughout the year. However, as only young expanding leaves are susceptible, the infection period runs from spring (October-November) until autumn (April-May). From the end of autumn until the beginning of spring there is generally very little susceptible tissue available. Continuous high relative humidity or free moisture contribute to fruit-body maturation and ascospore release and thus to rapid build-up of inoculum (Park 1988; Park & Keane 1987), and in locations with high regular rainfall, such as the central North Island, conditions can be highly favourable for these fungi. After the establishment of infection the initial symptoms take about 3 weeks to appear. It takes 8-10 weeks for a leaf lesion to fully mature and produce ascospores, which are capable of causing fresh infection. In long dry periods this maturing process can take longer. The optimum temperature for infection to take place is 18°-24°C.

Fig. 14: Leaf of Eucalyptus delegatensis infected with Mycosphaerella cryptica

Fig. 15: Mycosphaerella cryptica severely infecting leaves of Eucalyptus delegatensis
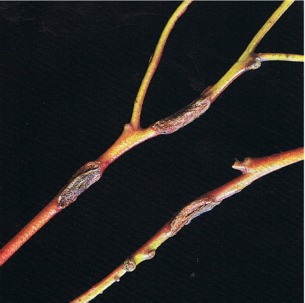
Fig. 16: Cankers on twigs of Eucalyptus delegatensis caused by Mycosphaerella cryptica
Mycosphaerella nubilosa
Mycosphaerella nubilosa forms creamy-yellow to pale brown irregularly shaped lesions on the leaves (Fig. 17). These lesions measure 5-25 mm in diameter and may coalesce. Juvenile foliage can be severely attacked. Adult foliage is mainly resistant, although infection may occur in the seasons during and immediately after transition. In the later stages of development the lesions become a grey-black colour on the undersurface because of the presence of the perithecia. With a few exceptions the host range is different from that of M. cryptica.

Fig. 17: Leafspots on Eucalyptus globulus caused by Mycosphaerella nubilosa
Mycosphaerella swartii
Mycosphaerella swartii forms small spots with a distinctive purple-red margin, which are scattered thickly over both upper and lower leaf surfaces. The teleomorph is rarely seen —fruit-bodies of the anamorph, Sonderhenia eucalyptorum (Fig. 18), are much more common and are submerged in the necrotic tissue. In humid weather, dark-brown conidiospores are exuded from the fruit-bodies and appear as minute dots on the leaf surface. The conidiospores are dispersed by water-splash. The main infection period is from February to May. This fungus has only rarely been associated with any defoliation.
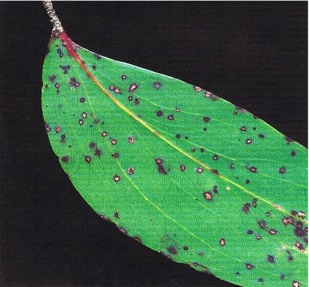
Fig. 18: Eucalyptus delegatensis showing leafspots caused by Sonderhenia eucalyptorum
Phaeothyriolum microthyrioides
The shield-shaped, minute, black fruit-bodies of Phaeothyriolum microthyrioides resemble iron filings, and are entirely superficial (Fig. 19). Discoloration of the leaf lamina below the fruit-bodies may occur. Ascospores are wind-dispersed and infection is mainly in the lower crown on mature foliage.
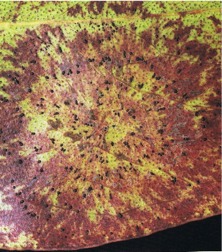
Fig. 19: A Phaeothyriolum microthyrioides leafspot on Eucalyptus fastigata, showing leaf discoloration and black fruit-bodies
Pseudocercospora eucalyptorum
First symptoms induced by Pseudocercospora eucalyptorum appear on the flush of new leaves in about January (Fig. 20). Once infection is established, a stroma made up of fungal hyphae is formed under the epidermis, and hyphae bearing conidiospores push up through the leaf. Conidiospores are wind-dispersed. The main infection period is from January to March.

Fig. 20: Leafspots caused by Pseudocercospora eucalyptorum on Eucalyptus regnans
Phaeophleospora eucalypti
Phaeophleospora eucalypti causes characteristic symptoms; initial pale-yellow blotches (Fig. 21) on the infected leaves change to a bright carmine red colour. As the tissue dies it becomes dark-brown. Soon after establishment of infection, fruit-bodies are formed under the epidermis and exude a mass of brown conidiospores on to the leaf surface. The conidiospores are dispersed by rain splash. The main infection periods are during spring and autumn. Both juvenile and adult foliage is susceptible to infection and heavily infected leaves are readily cast. Warm humid conditions favour the spread of the disease, and young E. nitens growing in such conditions may exhibit severe defoliation.

Fig. 21: Pale yellow blotches on Eucalyptus nitens leaves caused by Phaeophleospora eucalypti
Trimmatostroma bifarium and T. excentricum
Lesions caused by Trimmatostroma bifarium (Fig. 22) and T. excentricum are generally circular and spreading, and may be very similar in appearance to those caused by Aulographina eucalypti. Conidiospores are formed on the surface of the lesions in black powdery masses that are frequently arranged in a circular pattern. They are dispersed by wind and are found throughout the year.

Fig. 22: Leafspots caused by Trimmatostroma bifarium on Eucalyptus regnans
Barron Road Syndrome
Barron Road Syndrome (so called because of the location of the first study site), a condition which affects some of the ash eucalypts, e.g., E. delegatensis and E. regnans, is characterised by the abscission of new foliage, with the upper crown of badly affected trees gradually becoming totally devoid of leaves. Emerging leaves exhibit small necrotic spots with shoots, stems, and petioles often roughened with small galls. Older retained leaves may also be distorted and exhibit extensive leaf spotting and galling. A suite of fungi have been found associated with the affected tissues of young trees — these include Aulographina eucalypti, Elsinoe eucalypti, Mycosphaerella cryptica, M. swartii, Pseudocercospora eucalyptorum, and a Colletotrichum sp. As the trees age, the variety of fungi on leaves and shoots declines and A. eucalypti dominates.
NZ distribution:
Pseudocercospora eucalyptorum, Sonderhenia eucalyptorum, Phaeothyriolum microthyrioides, Mycosphaerella cryptica, and M. nubilosa are found throughout New Zealand. Aulographina eucalypti has been recorded throughout the North Island and is also present in Nelson and Westland. Phaeophleospora eucalypti is found in the North Island and Westland, and Trimmatostroma bifarium and T. excentricum are found in the central North Island, Manawatu, Nelson, and Westland. All fungi are common in the areas in which they are present and the first records in New Zealand were: Mycosphaerella cryptica, 1955; Phaeothyriolum microthyrioides, 1972; Trimmatostroma excentricum, 1978; Pseudocercospora eucalyptorum, 1980; M. nubilosa, 1981; T. bifarium, 1981; Aulographina eucalypti, Phaeophleospora eucalypti, 1981; Sonderhenia eucalyptorum, 1982.
Economic impact:
Many fungi infecting foliage of eucalypts have been recorded in New Zealand and recent years have seen a steady increase in the number of taxa. The close proximity of New Zealand to Australia, and the volume of trade between the two countries, ensure that the slow influx of the associated insect pests and diseases will continue. The vulnerability of the eucalypt plantations in this country to severe outbreaks of disease is generally related to how well the species are matched to their new environment, to stand management, and to the genetic origin of the planted material. The leaf spot fungi are frequently present at low levels, primarily affecting the older leaves in the lower crown, and in such instances do not have any significant impact on overall tree health. Under certain circumstances however, some of these fungi may be seriously detrimental to tree health. Fungal infection is more important when twigs, branches, and developing leaves become infected. Few studies have attempted to quantify growth loss caused by constant defoliation and none have carried out evaluations over the length of a rotation. Table 1, which has been drawn from a report from South Africa, illustrates the effect of consistent and severe defoliation on growth increment of young trees.
Table 1: Growth loss associated with different levels of defoliation of 4-year-old E. nitens caused by leaf-infecting fungi (Lundquist 1987)
| Defoliation (%) | Loss of potential growth increment (%) | ||
| Height | Diameter | Volume | |
| 0-12 | 0 | 0 | 0 |
| 12-25 | 0 | 0 | 0 |
| 25-38 | 0 | 0 | 0 |
| 38-50 | 2 | 8 | 17 |
| 50-63 | 18 | 24 | 37 |
| 63-75 | 47 | 48 | 64 |
| >75 | 91 | 81 | 99 |
Mycosphaerella cryptica and M. nubilosa have caused significant damage in areas of high rainfall and on highly susceptible species (E. delegatensis, E. regnans, and E. nitens), particularly affecting form in the first years of tree growth. In spite of an initial high level of interest by the forestry sector in E. delegatensis in the 1960s, increasingly poor performance due to infection by Mycosphaerella cryptica ensured that this species fell into disfavour. Mycosphaerella cryptica (thought at the time to be M. nubilosa) was first reported at epidemic levels in commercial eucalypt forests in the central North Island in 1971. Over 1000 ha of largely E. delegatensis had been severely affected, the symptoms including leaf blotch and defoliation, twig cankers, tip dieback, and stem malformation. Dieback and cankering of twigs stunts the growth and results in a bushy habit and multileadering. Juvenile foliage of E. nitens is also very prone to infection when this species is planted in areas that are warmer and at lower altitude than its natural range.
Defoliation of E. globulus ssp. globulus, E. nitens, and related species by M. nubilosa also occurred in the central North Island but tended to be overshadowed by the more visible and readily identifiable destruction by insect defoliators (all imports from Australia without natural predators) including the tortoise beetle (Paropsis charybdis).
Trees badly affected by Aulographina eucalypti may have over 90% of their leaf area covered with lesions. In New Zealand this level of infection has been seen in E. regnans in the central North Island and in E. delegatensis stands in the southern end of Kaingaroa Forest. Aulographina eucalypti has been associated with near-total defoliation of large stands of E. regnans, E. obliqua, and E. nitens at different times in Tasmania and Victoria.
Phaeophleospora eucalypti caused considerable defoliation of E. nitens in the 4-year period immediately after its introduction to New Zealand; disease levels subsequently dropped and in most locations remained at an acceptably low level. Since the establishment of an effective biocontrol agent for the defoliator Paropsis charybdis, increased plantings of E. nitens during the 1990s have been followed by an increase in levels of P. eucalypti infection. This has been particularly apparent in those areas where the climate is warmer and wetter than would be found in the natural range of this host. Of the several host species recorded in New Zealand, E. nitens is the most severely affected. The disease does not appear to be of any significance in Australia.
Barron Road Syndrome particularly affects Eucalyptus regnans- other species (E. fastigata, E. delegatensis in particular) may exhibit some of these symptoms but generally retain most of their foliage. Plantations in the central North Island where rainfall can be in excess of 2000 mm have been most severely affected, with the humid gully plantings exhibiting the highest disease levels. An extremely wet spring in 1989-90 led to massive defoliation of several thousand hectares of E. regnans, whilst adjacent stands of E. fastigata, although spotty, retained most of their foliage. Decline of E. regnans and E. delegatensis, characterised by poor growth, crown dieback, and some mortality, in the larger plantations in the central North Island has been ongoing since the early 1980s. Both species originate from areas in Australia with a distinct winter rainfall pattern. Although rainfall data for the affected New Zealand locations shows that from 1971 through to summer 1979 there were consistent winter rainfall maxima, subsequent uniform rainfall distribution patterns have occurred with some summer peaks. Transfer of eucalypt species from winter to summer rainfall areas is usually unsuccessful but this lack of adaptability may have been disguised in the 1970s. Eucalyptus fastigata, planted alongside the Barron Road Syndrome-affected E. regnans and generally unaffected (extensive leaf spotting but little foliage loss), comes from an area with a seasonally uniform rainfall pattern. Consequently it is better adapted to withstand the onslaught of fungal attack occurring when temperatures are moderate to warm and there is plenty of moisture available.
Control:
Tasmanian provenances of E. delegatensis and E. regnans are more resistant to attack by the Mycosphaerella spp. than those from Victoria. The potential for selection of resistant genotypes to reduce the effects of Mycosphaerella spp. has been examined but without commitment in New Zealand and the response to the epidemics of the 1960s and 70s was to shift the focus and choose other species of eucalypts for the affected areas. Eucalyptus regnans and E. fastigata followed E. delegatensis as the ash eucalypts of choice in the wetter areas of the North Island. There appears to be potential for selection of trees showing some resistance to Phaeophleospora eucalypti.
Biocide trials carried out on young E. regnans showed that regular (3-4 weekly) applications of a broad-spectrum fungicide gave reasonable control of Barron Rd Syndrome. As spray schedules are not a practical option for control of disorders in plantation forestry, the immediate strategy was to make a shift in species/ site matching, with E. regnans no longer planted in locations where this species was badly affected. In 1991, 312 families of E. regnans were planted in a multi-purpose trial, which included the intention of determining family susceptibility to Barron Rd Syndrome. This trial was sited outside the area where the syndrome was most severe and, as there was no occurrence of the disease, no evaluations could be made. Unexpectedly, in the autumn of 1994 an outbreak of M. cryptica, which caused substantial dieback, resulted in family identification of varying levels of resistance to this disease.
Chemical control of Mycosphaerella spp. has been necessary in forest nurseries where there is an inoculum source nearby; fortnightly applications of chlorothalonil (3.4 kg in 1000 //ha) have been shown to control the disease. Chlorothalonil also controls Barron Rd Syndrome, at least on an experimental scale. Substantial reduction in levels of Phaeophleospora eucalypti infection of E. nitens was achieved in trials with a range of fungicides applied at fortnightly intervals. Control of the other leaf spot fungi has not been attempted.
Strategies for managing disease in eucalypt plantations in New Zealand continue to focus on accurate matching of species with site requirements. Provenance trials of preferred species are in place in a number of locations; however, susceptibility to disease, although evaluated, does not tend to be the primary objective; determination of growth and form take priority.
References:
Cheah & Hartill 1987; Dick & Gadgil 1983; Fry 1983; Kay 1993; Lundquist 1987; Ministry of Forestry 1993; Park 1988; Park & Keane 1987; RevelI 1981 ;Turnbull & Pryor 1984; Wall & Keane 1984, Weston 1957.
This information is intended for general interest only. It is not intended to be a substitute for specific specialist advice on any matter and should not be relied on for that purpose. Scion will not be liable for any direct, indirect, incidental, special, consequential or exemplary damages, loss of profits, or any other intangible losses that result from using the information provided on this site.
(Scion is the trading name of the New Zealand Forest Research Institute Limited.)

 Farm Forestry New Zealand
Farm Forestry New Zealand

