PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Melampsora leaf rusts of poplar
Scion is the leading provider of forest-related knowledge in New Zealand
Formerly known as the Forest Research Institute, Scion has been a leader in research relating to forest health for over 50 years. The Rotorua-based Crown Research Institute continues to provide science that will protect all forests from damage caused by insect pests, pathogens and weeds. The information presented below arises from these research activities.
Melampsora leaf rusts of poplar,
Forest Pathology in New Zealand No. 20.
Based on A.G. Spiers (1990)

Fig. 1 - Trees of Populus 'Generosa' with necrotic, withered foliage as a result of severe rust attack by Melampsora larici-populina . Populus 'Generosa' is highly susceptible to M. larici-populina and many trees of this hybrid have died as a result of repeated defoliation.
Causal organisms
Melampsora larici-populina Klebahn; Melampsora medusae Th?men, Melampsora ×medusae-populina Spiers
Type of injury
Spotting of leaves which can cause premature defoliation and lead to a reduction in growth. Tree death may occur after several years' severe infection (Fig. 1).
Diagnostic features
- Small orange-yellow pustules on one or both leaf surfaces (Fig. 2).
- In severe attacks, pustules cover both sides of leaves giving trees a golden appearance.
- Heavily infected leaves turn brown, wither, and curl at the margins before failing.
- In late summer, small amber/brown-to-black raised spots form on both leaf surfaces.
- The two Melampsora species and the hybrid can be distinguished microscopically by spore ornamentation.
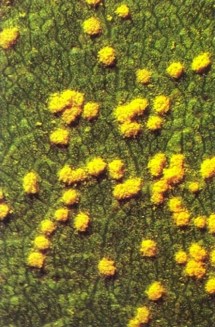
Fig. 2 - Rust pustules (uredinia) of M. medusae on a highly susceptible cultivar of P. nigra 'Italica'.

Fig. 3 - Poplar leaf rust ( Melampsora medusae) on P. x euramericana '178'.
Hosts
At the time of the arrival of M. larici-populina and M. medusae in New Zealand in 1973, the most widely planted poplars were a range of cultivars of Populus x euramericana and these proved highly susceptible to both the rust species (see table 1). These cultivars are now uncommon through much of the country though they may be found in drier areas.
In addition to the poplar hosts Melampsora medusae has been occasionally found on Larix decidua and Melampsora larici-populina on Larix decidua, L. kaempferi, and rarely on Pinus radiata. Melampsora ×medusae-populina a hybrid of the two species, is thought to have arisen in Australia, was first recognised in New Zealand in 1991 and has a host range similar to that of M. medusae.
Distribution
Both poplar leaf rusts entered New Zealand in 1973 via trans-Tasman wind currents from Australia. Melampsora larici-populina spread rapidly over the next few years and is now present throughout the country. It is more prevalent in moist areas of New Zealand and has little effect on even the most susceptible poplar cultivars in the drier regions of the South Island, viz Marlborough, Canterbury, and Otago. Melampsora medusae remained confined to some isolated trees and plantations of P. deltoides and had apparently died out by 1984, presumably because of a lack of suitable overwintering hosts. However, an outbreak of the disease was recorded in Hamilton in February, 1990. The hybrid species M. ×medusae-populina has not been recorded outside Northland and it is possible that it has not survived because of a lack of suitable hosts.
Disease development
The Melampsora rusts produce several different spore stages in sequence through the growing season to complete their life-cycle (Fig. 4). The orange-yellow pustules contain masses of urediniospores which are blown over great distances and infect other poplars. A new crop of spores is produced approximately every 2 weeks, depending on weather conditions. With deciduous poplars, infection ceases once all the leaves have fallen. On semi-evergreen poplars,
which do not shed all their leaves, the fungus can persist and continue to produce spores all through winter and spring. These hosts, therefore, provide a ready source of inoculum for the deciduous poplars as they flush in spring. On older infected leaves, another type of spore - the teliospore - is produced. These are borne in small amber-brown to black spots and remain dormant on fallen leaves until the following spring when they germinate to produce basidiospores. The basidiospores can only infect the needles of conifers and on these hosts, pycniospores and aeciospores are formed (Fig 5). Wind-borne aeciospores re-infect emergent poplar foliage in spring, resulting in the production of urediniospores again to complete the fungal life-cycle.
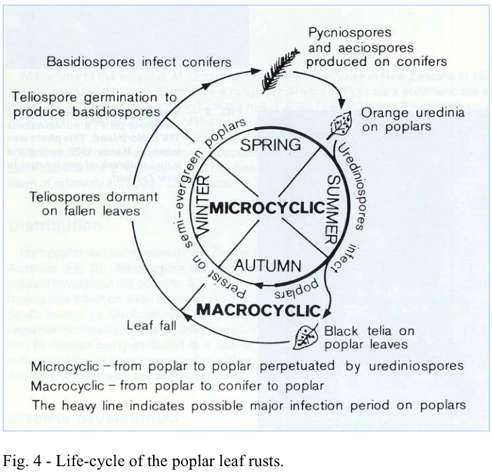
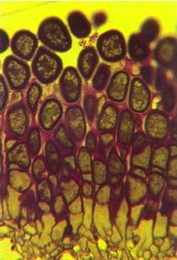
Fig. 5 -Vertical section through an aecidium of M. larici- populina on a larch leaf showing how aeciospores are formed in columns and tightly packed.
The poplar rust fungi do not require completion of the full life-cycle for survival; infection can cycle on from poplar to poplar by urediniospores alone. Infection of the conifers, however, is dependent on infected poplars, and spread from conifer to conifer does not occur.
Free water on the leaf surface is required for urediniospore germination and host infection. Regular rainfall (at 7- to 12-day intervals) provides optimum conditions for re-infection and the build-up of rust inoculum.
Economic importance
The most common use of poplars in New Zealand has been as shelterbelts on horticultural land and farmland. However, they have also been widely used for soil conservation purposes and in forestry. The following damage can occur on poplars after repeated defoliation:
- delayed and reduced spring flushing;
- reduced height and diameter growth, and reduced root development;
- distorted shoot growth;
- increased susceptibility to other fungal pathogens;
- reduced hardening off and thus more frost damage;
- dieback and death.
The demise of the semi-evergreen poplar ( P. nigra 'Sempervirens'), which allowed urediniospores to overwinter, has meant that the inoculum build-up in early spring is considerably delayed. Most poplars can, therefore, grow a reasonable amount before the disease causes complete defoliation. Nevertheless, the severity of annual rust infection varies markedly, depending on the time of initial infection, presence of alternate hosts, locality, and attendant climatic conditions.
Rust infection of Pinus radiata is very uncommon and of no significance in New Zealand. Larches are frequently infected (Fig. 6 and 7) where they are grown in association with poplars, but damage is restricted to some minor leaf fall.

Fig. 6 - Larch leaf bearing pycnidium of M. larici-populina . The pycnidium has exuded a nectar droplet to attract insects.
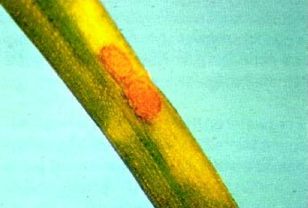
Fig. 7- Aecia of M. larici-populina containing thousands of aeciospores formed on a larch leaf.
Control
Planting rust-resistant poplar varieties is the best means of combating poplar rust. It is advisable to use a mixture of varieties to ensure genetic diversity rather than rely on one single cultivar. At the time of arrival of M. larici-populina and M. medusae in New Zealand (1973), most widely planted cultivars had narrow genetic base and they proved to be highly susceptible to both rust species.
Since 1973 some 239 new clones and 290 seedlots of several species have been imported from Europe, Asia, and America. Some of this material has been hybridised with local selections to give a range of rust-resistant cultivars. Some of these are included in Table 1.
Selection for rust resistance has been complicated by the appearance of different races of M. larici-populina. The rust resistance of certain cultivars has varied between locations, years, and even within the same seasons, depending an which race is dominant. Dominant races have disappeared over winter, hence the advisability of planting clonal mixtures.
Chemical control can be practicable for orchard shelterbelts. Options include triadimefon (0.05%), copper oxychloride (0. 1%) or dodine (0.1 %) and should be applied when the first rust pustules are seen, and continued at 3-weekly intervals. This time period can be extended during dry weather. There is no need for control of the disease in the conifer hosts.
TABLE 1 -SUSCEPTIBILITY OF POPLARS TO MELAMPSORA SPP.
|
Species |
Clone |
Melampsora larici-populina |
Melampsora medusae |
Melampsora medusae-populina |
|
P. alba |
|
Resistant |
Resistant |
|
|
P. alba |
'Pyramidalis' |
Resistant |
Resistant |
|
|
P. alba |
‘BO/2’ |
Resistant |
Resistant |
|
|
P. alba |
‘I59/1’ |
Resistant |
Resistant |
|
|
P. alba x glandulosa |
‘Yeogi 1’ |
Resistant |
Resistant |
Resistant |
|
P. alba x glandulosa |
‘Yeogi 2’ |
Resistant |
Resistant |
|
|
P. x canescens |
|
Resistant |
Resistant |
|
|
P. deltoides |
‘Frimley’ (old N.Z clone) |
Slightly susceptible |
Susceptible |
Slightly susceptible |
|
P. deltoides x maximowiczii |
‘Eridano’ |
Resistant |
Resistant |
Resistant |
|
P. deltoides x trichocarpa |
‘Generosa’ |
Highly susceptible |
|
|
|
P. deltoides x yunnanensis |
‘Kawa’ |
Resistant |
Resistant |
|
|
P. x euramericana |
‘Flevo’ |
Resistant |
Resistant |
Slightly susceptible |
|
P. x euramericana |
‘I 30’ |
Highly susceptible |
Susceptible |
Highly susceptible |
|
P. x euramericana |
‘I 78’ |
Highly susceptible |
Susceptible |
|
|
P. x euramericana |
‘I 154’ |
Resistant |
Resistant |
|
|
P. x euramericana |
‘I 214’ |
Susceptible |
Susceptible |
|
|
P. x euramericana |
‘I 455’ |
Susceptible |
Susceptible |
|
|
P. x euramericana |
‘Laevigata’ |
Susceptible |
Susceptible |
|
|
P. x euramericana |
‘Luisa Avanzo’ |
Resistant |
Susceptible |
|
|
P. x euramericana |
‘Robusta PH’ |
Susceptible |
Susceptible |
|
|
P. x euramericana |
‘Serotina’ |
Highly susceptible |
Susceptible |
|
|
P. x euramericana |
‘Serotina Aurea’ |
Highly susceptible |
Susceptible |
|
|
P. x euramericana |
‘Tasman’ |
Resistant |
Resistant |
Slightly susceptible |
|
P. x euramericana |
‘Veronese’ |
Resistant |
Resistant |
|
|
P. x euramericana |
‘Triplo |
Susceptible |
Susceptible |
Highly susceptible |
|
P. x gileadensis |
‘Candicans’ |
Variable† |
Resistant |
|
|
P. maximowiczii x trichocarpa |
‘Androscoggin’ |
Variable† |
Susceptible |
|
|
P. nigra |
‘Italica’ |
Highly susceptible |
Susceptible |
|
|
P. nigra |
‘Sempervirens’ |
Highly susceptible |
Resistant |
|
|
P. nigra x ‘Regenerata’ |
‘Eugenei’ |
Highly susceptible |
Susceptible |
|
|
P. tremula |
|
Resistant |
Resistant |
|
|
P. tremuloides |
|
Resistant |
Resistant |
|
|
P. trichocarpa |
‘PMC 471’ |
Resistant |
Resistant |
|
† Depends on race of the fungus
Bibliography
Spiers, A.G. 1976: Fungicides for control of poplar leaf rust and effects of control on growth of Populus nigra cv. 'Sempervirens' and P. x euramericana cv. '1-214'. New Zealand Journal of Experimental Agriculture 4: 249 - 254.
Spiers, A.G.; Hopcroft, D.H. 1994: Comparative studies of the poplar rusts Melampsora medusae and M. larici-populina and their interspecific hybrid M. medusae-populina. Mycological Research 98: 889-903.
Van Kraayonoord, C.W.S.; Laundon, G.F.; Spiers, A.G. 1984: Poplar rusts invade New Zealand. Plant Disease Reporter 58: 423-427.
Wilkinson, A.G. 1987: The return of the poplar. Ministry of Works and Development, Streamland 52.
Wilkinson, A.G.; Spiers, A.G. 1976: Introduction of the poplar rusts Melampsora larici- populina, M. medusae to New Zealand and their subsequent distribution. New Zealand Journal of Science 19: 195-198.
Compiled: 1990; revised 2010
This information is intended for general interest only. It is not intended to be a substitute for specific specialist advice on any matter and should not be relied on for that purpose. Scion will not be liable for any direct, indirect, incidental, special, consequential or exemplary damages, loss of profits, or any other intangible losses that result from using the information provided on this site.
(Scion is the trading name of the New Zealand Forest Research Institute Limited.)

 Farm Forestry New Zealand
Farm Forestry New Zealand

