PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Field Assessment, Control and Identification of Common Foliage Diseases of Pine in New Zealand
Lindsay Bulman and Judy Gardner, June 2014.
(You can also download this field guide as pdf 6.6mb)
Objectives
This guide provides information for foresters to identify common foliage diseases of pines and list available methods of control. Specifically,
- Symptoms of the four common pine foliage diseases are described and shown
- The period of symptom expression and commonly affected regions for each disease aree provided
- Control measures and recommendations for each disease are listed
Background
In parts of New Zealand, shelterbelts, woodlots, and commercial plantations suffer from Dothistroma needle blight; Cyclaneusma needle cast; physiological needle blight (PNB); or red needle cast (RNC). In some regions all four diseases are present. Seasonal development of these diseases differs between diseases, locations, and seasons and years. Many factors, some still to be confirmed, contribute to outbreaks (for instance rainfall, temperature, tree age, silvicultural treatment).
Dothistroma needle blight can be controlled by applying a copper fungicide in October or November. Timing depends in the location and or season with spray applied earlier in warmer areas. Cyclaneusma is more difficult to control. Susceptible trees can be removed during thinning operations if thinning is undertaken when the disease is showing and the thinning crew can recognise the disease. Red needle cast and PNB might be controlled by application of a phosphite spray and research is underway to determine this.
Control of needle disease is dependent on the correct identification of the disease. For instance, applying copper spray to control Cyclaneusma needle cast is a waste of time and resources. The aim of this guide and the key to identifying needle diseases is to enable farm foresters and other forest growers to be able to identify a needle disease with confidence.
Field Assessment
Process
There is a process that should be followed when assessing foliage diseases:
- Agree on the purpose of the assessment
- Identify the disease correctly
- Assess trees consistently using the most appropriate method
- Be aware of conditions
Details on disease identification are provided later in this guide. It is vital that the disease is correctly identified because control methods vary for different diseases. Disease symptoms differ at different times of the year and on different hosts and assessors should be aware of that.
Assessments are carried out for a number of reasons and the purpose will influence the assessment method chosen. For instance, if one wishes to report an overall level of disease a rating of absent, low, medium, or high may be sufficient. However, if one wants more accurate data on which to make a decision on whether to undertake control measures or not a more sensitive estimate of disease would be needed.
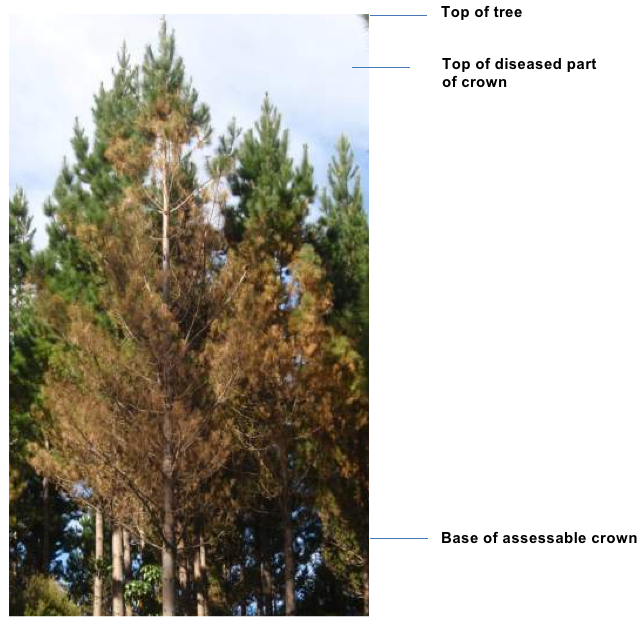
Conditions will also influence assessment. Large trees are more difficult to assess because often it is difficult to get an unobstructed view of the crown. Large trees are more prone to natural needle death, particularly in the lower part of the crown, due to suppression and lack of light. It can be difficult differentiating between defoliation due to suppression and that due to infection by pathogens. Aspect can influence disease assessment because light, prevailing wind, inoculum source, proximity of neighbouring trees can differ from one side of a tree to the other. Likewise, assessment scores may vary depending on the amount of light or the direction the assessor is looking. It is more difficult to see diseased foliage when looking into the brightest part of the sky and easier to see disease when the brightest part is behind.
Assessment
The objective of any assessment is to estimate the amount of disease in the tree crown. It is important to assess only current infection. Therefore, bare branches caused by needles lost from past disease, regardless of cause (i.e. red needle cast) are ignored (see Fig 2 below).
Disease level on individual trees is assessed as the percentage of the total unsuppressed foliage present on the tree that is diseased. The proportion of infected to uninfected foliage is estimated and given as a percentage in 5% steps, i.e., 5, 10, 15, 20, 25, etc. A score of 40% indicates that 40% of the foliage present is diseased.

The assessment method described above is the method which is most commonly used. The annual aerial survey for Dothistroma needle blight undertaken by many forest owners in New Zealand is based on this method. An evaluation of the accuracy of the assessment is provided by van der Pas, Kimberley, and Kershaw (1984).
In a young plantation, before canopy closure, all foliage of the tree contributes to tree growth and therefore should be assessed. In closed-canopy stands, only the unsuppressed part of the crown (the part of the crown above the canopy closure points) of a tree should be assessed.
Assessment of severity of infection on individual trees is based on the observation that the pathogen usually infects and causes defoliation of the lower part of the crown first.
The number of trees that should be assessed during a ground based inspection will vary depending on the accuracy needed. Sometimes, where to assess is just as important as what to assess. For instance, disease levels in a moist gully may be much higher than in the remainder of the plantation. In order to obtain an accurate estimate of the amount of disease in a plantation variation due to microsite (due to topography, terrain, and stand treatment) needs to be considered and planned for. Once the variation within a plantation is understood an average of about 50 trees is usually sufficient to get a reliable estimate.
Control options
One of the main aims of disease assessment is to determine if disease has reached a level that warrants some form of treatment – what is termed the intervention threshold. The threshold varies depending on the value of the trees, the disease and the cost of control. If trees are valuable then the intervention threshold will be lower than that of less valuable trees. Different diseases cause different levels of growth loss, i.e. trees with 30% of their foliage affected by Dothistroma needle blight will lose more growth than trees affected by Cyclaneusma needle cast at the same levels.
As a rule of thumb, the following table shows appropriate intervention levels for each of the four diseases based on their effect on growth.
Threshold disease levels that warrant intervention (% of diseased foliage)*
| Disease | Pruned stand | Unpruned stand | Intervention |
| Dothistroma | 20 | 25 | Spray with cuprous oxide |
| Cyclaneusma | 35 | 45 | Remove diseased trees during thinning |
| Red needle cast | 30 | 40 | Spray with phosphite |
| Physiological needle blight | 35 | 45 | Spray with phosphite |
*Assuming produce from the pruned stand is sold at a premium to the unpruned stand
 |
 |
| 1% of foliage diseased |
5% of foliage diseased |
 |
 |
| 20% of foliage diseased |
20% of foliage diseased |
 |
 |
| 35% of foliage diseased |
65% 0f foliage diseased |
 |
 |
| 70% of foliage diseased | 95% of foliage diseased |
Fig 4 – Trees with various levels of dothistroma needle blight severity
Red needle cast and physiological needle blight
Chemical control options for red needle cast are still being developed. Provisional results of research carried out to date indicate that phosphite is a potentially effective chemical to control red needle cast. Efficacy and persistence increase as dose increases but the optimum dose and timing of application still need to be worked out.

Cyclaneusma needle cast
Chemical control of cyclaneusma needle cast is possible, but it is not economic. One would have to apply chemical at least 3 or 4 times in one season and the spray cost would not be recovered by the extra volume produced. Instead, owners of small plantations or woodlots should be able to control the disease by taking advantage of the fact that trees tend to be either very susceptible to the disease or not (Fig 3). The disease usually first appears when trees are about 6 years old, so if thinning is carried out when symptoms are most apparent (around spring time) highly susceptible trees can be selected and removed.
Dothistroma needle blight
The photos on the following page show young trees with different levels of dothistroma needle blight. The top left tree looks healthy but is given a score of 1% because it is extremely rare for a tree to be free of disease. At the other extreme the tree at the bottom right has almost all of its foliage diseased and is scored at 95%. All trees on the top row do not warrant control measures because disease levels are too low – the cost of control would not be recovered by the additional increment gained.
At present, only dothistroma needle blight is controlled operationally. Copper spray is applied when average disease levels reach 20%, but some companies take a risk adverse approach and spray at lower disease levels. Stands are generally sprayed 3 or 4 times before they reach the age of 15 years, after that natural resistance to dothistroma occurs.
The Dothistroma Control Committee is responsible for organising spray operations, specifically:
- Buying bulk lots of copper fungicide and spray oil at competitive rates;
- Organising and letting contracts for the aerial application of the fungicide;
- Monitoring the quality of the fungicide;
- Reviewing any new techniques or developments from research.
The Committee comprises representatives from the New Zealand Forest Owners’ Association, Ministry for Primary Industries, Scion, and the Farm Forestry Association. The Secretary of the Dothistroma Control Committee can be contacted at the following address:
Dothistroma Control Committee
P. O. Box 1035
Rotorua.
Also download the guide on the assessment and control of dothistroma needle blight.
Symptoms associated with the four needle diseases of Pinus radiata
See key to identifying needle diseases »
| Symptom | Cyclaneusma needle cast | Physiological needle blight | Red needle cast | Dothistroma needle blight |
| Time of year expressed | September to November | June to November | April to October | All year, first appears on current foliage about December |
| Incidence and severity | Scattered individuals, up to 90% severity on very susceptible trees | Localised distribution, very high incidence in affected parts of a stand | Localised/general distribution, almost every tree in affected parts of a stand | General distribution, almost every tree in affected parts, but tree to tree variation is apparent. |
| Needle colour | Yellow, then gold, then brown | Red, then red-brown, then grey | Oily green band, then yellow, then red | Brick red bands on green needles with black spots usually seen within the bands. |
| Needle wilt | No wilt | Wilt common at late stage of disease development | No wilt | Needles may wilt, but usually wither and turn brown/grey. |
| Needle retention | Needles detach very readily | Needles retained | Needles detach readily | Needles die completely and are retained. |
| Cambium and bark | No damage, no lesions, no resin | No damage, lesions, or resin | No damage, no lesions, resin blobs sometimes seen at needle base | No damage, no lesions, no resin |
| Tree age | Six to 20 years | Generally over 15 years | All ages, but generally over 3 years | From planting up to about 15 years |

 Farm Forestry New Zealand
Farm Forestry New Zealand

