PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Tree decays
Scion is the leading provider of forest-related knowledge in New Zealand
Formerly known as the Forest Research Institute, Scion has been a leader in research relating to forest health for over 50 years. The Rotorua-based Crown Research Institute continues to provide science that will protect all forests from damage caused by insect pests, pathogens and weeds. The information presented below arises from these research activities.
Forest Pathology in New Zealand No. 17,
Tree decays.
Revised 2009
Based on I.A. Hood (1986)
Introduction
Causal organisms
Type of injury
Diagnostic features
Hosts
Distribution
Disease development
Economic importance
Control
Table 1 - Decay fungi of both living native and introduced trees
Table 2 - Decay fungi of living native trees only
Bibliography

Fig. 1 - Stem of large rimu tree (Dacrydium cupressinum) broken by wind as a result of extensive butt heartwood decay caused by Armillaria sp.
Introduction
This leaflet describes decays of sapwood and heartwood in standing or fallen trees. Rots of stacked logs or processed timbers are excluded. Sapwood is the outer wood in which, in the growing tree, sap flow occurs. It contains both living and dead cells. Decay of sapwood is known as sap rot. Heartwood is the wood, sometimes darker in colour, present at the centre of an older tree. It contains no living cells, but may have natural preservative substances which provide resistance against most decay fungi. Trees in which the heartwood is decayed are said to have heart rot.
Causal organisms
Wood decay is caused by a wide range of fungi. The major decay fungi of living trees are listed in Tables 1 and 2. Most of these fungi also occur on dead trees, stumps, or logs of their hosts, and even in living trees they decay only the dead heartwood. However, a number are parasitic (indicated by an asterisk in Table 1) and may invade live tissues.
Type of injury
Decay and deterioration in strength and quality of sapwood or heartwood in living or dead, standing or fallen trees.
Diagnostic features
Early and intermediate decay (dozy wood): Early stages of decay are not always easy to detect without laboratory examination. Comparisons should be made with healthy wood. Indications may be given by:
- Red, brown, or white discoloration or staining. (Dark blue or black wedge-shaped stains are caused by non-decay fungi which reduce the visual quality but not the strength of wood.)
- A decrease in hardness (testable by prodding with sharp instrument), and relative ease of cutting during sawing (cut faces have a roughened texture).
- An even fracture without splintering when broken.
- Thin, dark coloured "zone lines" present in wood.
- Bark readily separates from wood (white fans of fungal mycelia may lie between).
- A mushroom-like odour.
- Advanced decay: Well-decayed wood is indicated by changes in colour, form, weight, and strength. Field identification of the causal fungus is sometimes possible at this stage, from a consideration of the following points:
- Presence of identifiable fungal fruiting bodies (not necessarily conclusive since they may be
produced by fungi other than those causing the rot). - Occurrence of a distinctive rot pattern, characteristic of a specific fungus, which tends to be the same regardless of host. Examples include white rots (Fig.2), brown, cubically-cracked rots (Fig. 3), and "pocket" rots in which the decayed wood is traversed by a system of cavities (Fig. 4, 5, 6).
- Restriction of decay to a particular habitat. For instance, many rot fungi only occur in dead
trees, stumps, or logs, while others also decay living trees (Tables 1, 2).
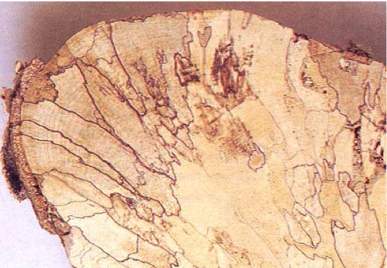
Fig. 2 - White rot in log of tawa (Beilschmiedia tawa) caused by Antrodiella zonata. Many zone lines have been produced.

Fig. 3 - Brown cubical rot in kauri (Agathis australis) produced by a native fungus related to Northern Hemisphere Phaeolus schweinitzii.

Fig. 4 - Fruiting bodies and characteristic honeycomb decay caused by Rigidoporus concrescens taken from heartwood in the butt region of rimu (Dacrydium cupressinum).
This decay leads to windthrow or stembreak of overmature trees.
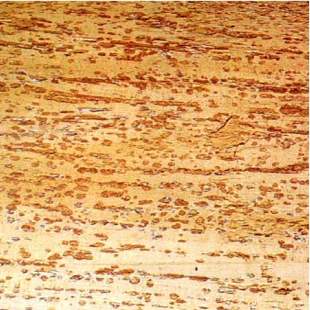
Fig. 5 - White rot of hinau (Elaeocarpus dentatus) with pockets containing mycelium of Phellinus wahlbergii.

Fig. 6 - Pocket heartrot of totara (Podocarpus totara) produced by Inonotus lloydii. This decay is known as kaikaka.
Hosts
All tree species are subject to fungal decay, but individual fungi vary in their ability to attack different hosts. Some fungi are capable of causing rots in many different hosts, while others are confined to particular groups, or even to single species. A number of decay fungi are largely restricted to "hardwood" or "softwood" trees, terms which do not necessarily refer to wood toughness. Hardwoods are broadleaved trees such as tawa, kamahi, eucalypts, and oak; softwoods are coniferous trees with needles or scale leaves such as rimu, kauri, pines, and larches. Several fungi are found mainly on introduced hosts, suggesting that they themselves may have been introduced to this country (e.g., Abortiporus biennis, Amylostereurn areolatum, Gloeophyllum sepiarium, Hapalopilus nidulans, Phaeolus schweinitzii. Table 1).
Distribution
Decay fungi are found in natural and planted forests throughout the country, and also occur wherever woody plants are grown, such as in gardens, orchards, and on farms. Native forests support the greatest number of species of decay fungi. Detailed distribution ranges of some rot fungi are poorly known but many appear to be widespread throughout New Zealand. A number of species are restricted by the limited distribution of their natural hosts. For example, Heterobasidion araucariae (Table 1) and a fungus related to the Northern Hemisphere species Phaeolus schweinitzii (Table 2) are not found outside the natural range of Agathis australis (kauri), and Grifola colensoi (Table 2) is found mainly in localities where Nothofagus species (native beeches) occur. On the other hand, species such as Fomes hemitephrus (Table 2, Fig. 18) occur on hardwoods in both beech and podocarp/hardwood forests.
Disease development
Wood decay fungi reproduce by means of spores released into the air from fruiting bodies. These fruiting bodies may take the form of discs, cups, brackets or shelves, crusts, toadstools, or jelly-forms. Decay begins when airborne spores land and germinate on recently dead or fallen trees, or on wood surfaces freshly exposed by the wounding of living trees. Entry wounds or openings are created by machines or falling trees during logging or thinning operations, destructive winds, fires, heavy snowfalls, physiological stresses induced by drought or cold, and animal damage (e.g., chewing or bark stripping by possums or kaka). Fungi can also invade trees through their roots or by first colonising dead branches or tops. Some fungi may be already present as endophytes within the sapwood of living branches but unable to grow until death and drying occur.
Decay fungi grow within wood in the form of microscopic branching threads, or hyphae, known collectively as a mycelium. As mycelia of different fungi extend, they come into contact with each other. If antagonisms occur, thin black zone plates form in the wood along the planes of contact between mycelia. These are visible as thin lines in cut surfaces and indicate the sphere of activity of each mycelium. Zone plates may also develop parallel to freshly cut surfaces and protect the mycelium from excessive drying. With time, successions occur, one fungus replacing another as wood decay proceeds.
The hyphae of wood decay fungi secrete enzymes as they grow. These destroy small sections of the wood cell walls, which consist primarily of cellulose and lignin. The mycelium is thus able to penetrate deeper into the wood, and in the process obtains nutrients from substances produced during the breakdown of the cell walls. Because each species of decay fungus secretes a characteristic complement of wood-destroying enzymes, distinctive decay patterns are produced. Thus, brown- rot fungi (Fig. 3) destroy the cellulose and hemicellulose components of wood cell walls, but are unable to degrade the lignin. The resultant decay has a structured appearance and is dark in colour due to the high proportion of lignin present. White-rot fungi are able to destroy lignin as well as cellulose, and the subsequent decay is paler in appearance and less structured (Fig. 2). Fungi forming pocket rots break down the lignin selectively in discrete zones within the wood, leaving "pockets" filled with white cellulose. Later the cellulose is also destroyed.
The process of decay is governed by a number of environmental factors. Like all organisms, decay fungi require an adequate water supply. Decay will not normally occur or be sustained in wood with a moisture content maintained below 20% (oven dry weight basis), although these conditions will not necessarily kill the decay organism once it has become established. In addition, decay fungi are aerobic and will not grow at low oxygen or high carbon dioxide concentrations. Most decays are thus also inhibited by a high wood-moisture content (exceeding about 70%), because this excludes air from the wood cells.
In living trees deposits of toxic chemicals that inhibit most decay fungi normally protect the heartwood from rotting. In many trees heartwood decay is also prevented by a naturally occurring high moisture content (e.g., in excess of 80% in species of Podocarpus compared to only 40-45% in Pinus radiata). Nevertheless, a limited number of decay fungi are able to tolerate these conditions and produce characteristic heart rots in their living hosts. Decay in the sapwood is usually prevented by the natural resistance responses of the living cells (e.g., resin flow in softwoods, following wounding) and by a high moisture content (exceeding 90% in most trees).
If decay is present it is generally localised at sites of wounding or stress. However, when a tree dies or fails, the sapwood rapidly begins to decay, whereas the heartwood initially remains relatively resistant to rot fungi. The decay of fallen trees is governed by their rate of drying which, in turn, is controlled by combinations of the following factors: tree species; stem diameter; proportion of sapwood; length of stem in ground contact; extent of uprooting; amount of shade cover; degree of stem shatter; location, and time of year.
Even though rots destroy wood, crown health is not usually affected because most of the decay fungi are saprophytes and feed only on dead tissue of living trees. However, some are parasitic fungi and may extend their activity into the still living sapwood. A limited number are capable of invading the host directly, without the need for wounding. These fungi do impair tree health and in severe cases even kill the host. Severe root rotting may also affect crown health and tree stability.
Economic importance
Decay losses result from wood destruction, and from uprooting or breakage of rot-weakened trees during strong winds (Fig. 1). The most extensive decay damage is found in old growth beech or podocarp/hardwood forests, because rots are more prevalent in older trees (Fig. 7). Now that logging in these native forests has declined, decay damage has become economically much less important. Harvesting is currently prohibited in native forest reserves, and is allowed on private land only when carried out sustainably. In these situations losses may result from the rapid development of destructive sap rots, accompanied by insect tunnelling, if fallen trees are not retrieved quickly.

Fig. 7 - Extensive heartwood decay at the base of a large silver beach tree (Nothofagus menziesii)

Fig. 8 - Destruction of timber by a brown cubical heart rot in larch (Larix decidua) following damage to a residual tree during thinning operations.
In forests of introduced tree species, standing trees are cut at a comparatively early age, and losses from decay are usually uncommon. However, significant heart rot has occurred in older stands of Larix decidua as a result of damage caused by earlier pruning and extraction thinning operations (Fig. 8). Decay associated with dead branches and pruning wounds is also a feature of some Eucalyptus stands. Severe storms and heavy snow periodically cause extensive uprooting or breakage in plantation forests, and immediate salvage logging is necessary to avoid losses from stain and decay fungi or insect activity. In past years, windthrow-related losses have occurred at Golden Downs Forest (1968, 2008), Ashley, Hanmer, Eyrewell, and Balmoral Forests (1975), and Kaingaroa Forest (1982).
Decay fungi form an integral part of the biota in both indigenous protection forests and exotic plantations. Because they are able to break down the biologically resistant cellulose and lignin molecules, they are one of the main agents responsible for the decomposition of dead trees and fallen debris. As this woody matter decays, stored nutrients are released and used by other organisms within the forest ecosystem. Bound energy is also metabolised and carbon is emitted as carbon dioxide.
Decaying trees have other ecological functions. Hollowed cavities in dead snags and rotting wood lying on the floor provide habitats for birds and other forest animals.
Control
In any native forests where salvage or selective logging is still practiced, careful felling and harvesting techniques will minimise damage and decay in residual trees. In plantations of introduced softwoods, control measures are normally not necessary, except that scar damage should be kept to a minimum during extraction thinning. Windthrown trees should be salvaged as quickly as possible. If delays are unavoidable, water sprays or water immersion should be used to maintain a high moisture content and reduce the incidence of degrade in freshly salvaged logs.
When pruning some hardwood species it may be necessary to take measures to prevent the entry of decay fungi through pruning wounds..

Fig. 9 - Fruiting bodies of Armillaria limonea.

Fig. 10 - Fruiting bodies of Rigidoporus aureofulvus.
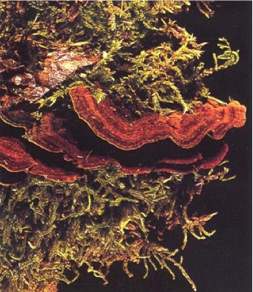
Fig. 11 - Fruiting bodies of Cyclomyces tabacinus.
Table 1 - Decay fungi of both living native and introduced trees (* Indicates parasitic fungus.)
FUNGI DECAYING BOTH HARDWOODS AND SOFTWOOD
| Name | Synonyms | Associated rot-type and comments |
| Armillaria limonea (G. Stev.) Boesew.* |
(Armillariella limonea) | White, large-pocket heart-butt rot; root disease of introduced trees (Forest Pathology in New Zealand 4). |
| Armillaria novae-zelandiae (G. Stev.) Herink* |
(Armillariella novae-zelandiae) | White, large-pocket heart-butt rot; root disease of introduced trees (Forest Pathology in New Zealand 4). |
| Chondrostereum purpureum (Pers.) Pouzar* |
(Stereum purpureum) | White rot; a fatal wound parasite of fruit trees (silver leaf disease); sap rot in pruned eucalypts |
| Cyclomyces tabacinus (Mont.) Pat.* |
(Inonotus tabacinus) | Yellow pocket heart-sap rot of damaged trees (Fig. 11). |
| Ganoderma applanatum sensu Wakef. |
White heart rot with brown zone lines (Fig. 12). | |
| Ganoderma australe (Fr.) Pat. |
(Fomes australis) (Elfvingia australis) |
White heart rot. |
| Gloeopeniophorella sacrata (G.Cunn.) Hjortstam & Ryvarden* | (Peniophora sacrata) (Phanerochaete sacrata) (Amylostereum sacratum) (Dextrinocystidium sacratum) (Gloeocystidiellum sacratum) |
Peniophora root and stem canker. White heart-sap rot; root disease especially of Pinus species (Forest Pathology in New Zealand 3). |
| Gymnophilus junonius (Fr.) P.D. Orton |
(Gymnopilus spectabilis) (G. pampeanus) |
On living Weinmannia racemosa and base of living Eucalyptus species; on stumps, Pinus species (Fig 13). |
| Ischnoderma rosulatum (G. Cunn.) P.K. Buchanan & Ryvarden |
(Grifola rosulata) (Polyporus rosulatus) |
Brown cubical heart rot in Larix decidua. |
| Junghuhnia vincta (Berk.) Hood & M. Dick* |
(Chaetoporus vinctus) (Poria vincta) (Rigidoporus vinctus) |
White rot; root disease of trees in shelterbelts and plantations in the Bay of Plenty. |
| Phaeolus schweinitzii (Fr.) Pat.* | Brown cubical butt rot; introduced, first found 1995; all records on Pinus radiata; distinct from a related fungus on Agathis australis. | |
| Phellinus gilvus (Schwein.) Pat. |
(Phellinus scruposus) (Fomes scruposus) |
White heart rot. |
| Pycnoporus coccineus (Fr.) Bondartsev & Singer |
(White rot; noted on living Knightia excelsa (Fig. 14). |
|
| Rigidoporus aureofulvus (Lloyd) P.K. Buchanan & Ryvarden | (Coltricia aureofulva) (Polyporus aureofulvus) |
White pocket heart rot with orange zone lines (Fig. 10). |
| Rigidoporus concrescens (Mont.) Rajchenb. |
(Polyporus catervatus, Tyromycetes catervatus) | Pocket "honeycomb" heart- butt rot, especially of Dacrydium cupressinum (Fig. 4). |
| Schizophyllum commune Fr.* |
(Coriolus versicolor) (Polystictus versicolor) |
Noted as a weak wound parasite on Sophora microphylla, Malus ×domestica. |
| Trametes versicolor (L.) Lloyd* |
(Coriolus versicolor) (Polystictus versicolor) |
Noted as a weak wound parasite on Betula pendula and fruit trees (Fig. 15). |
FUNGI DECAYING HARDWOODS ONLY
| Name | Synonyms | Associated rot-type and comments |
| Agrocybe parasitica G. Stev.* |
Heart rot; may also invade sapwood (Fig. 16). | |
| Phellinus robustus (P. Karst.) Bourdot & Galzin |
(Fomes robustus) | Heart rot. |
| Laetiporus portentosus (Berk.) Rajchenb. |
(Piptoporus portentosus) (Polyporus eucalyptorum) (Polyporus portentosus) |
Brown cubical heart rot of Nothofagus species; also on living Eucalyptus species |
FUNGI DECAYING SOFTWOODS ONLY
| Name | Synonyms | Associated rot-type and comments |
| Amylostereum areolatum (Chaillet ex Fr.) Boidin* |
White sap rot, mainly on introduced conifers; parasitic, transmitted by Sirex wood wasp (Forest and Timber Insects in New Zealand No. 20). |
|
| Heterobasidion araucariae P.K. Buchanan* | (Heterobasidion annosum var. araucariae) | Known only on Agathis australis (occasional sap rot of live tree) and Pinus taeda logs. |
| Stereum sanguinolentum (Alb. & Schwein.) Fr.* |
(Haematostereum sanguinolentum) |
Yellow stringy heart rot (and wood sap rot) of introduced conifers (e.g., Larix decidua) |

Fig. 12 - Fruiting bodies of Ganoderma applanatum sensu Wakef. in old stump.

Fig. 13 - Fruiting body of Gymnopilus junonius in living kamahi (Weinmannia racemosa).

Fig. 14 - Fruiting body of Pycnoporus coccineus from beneath.

Fig. 15 - Fruiting bodies of Trametes versicolor.

Fig. 16 - Fruiting bodies of Agrocybe parasitica in living tawa (Beilschmiedia tawa).

Fig. 17 - Fruiting body of Laetiporus portentosus from living red beech (Nothofagus fusca).
Table 2 - Decay fungi of living native trees only
FUNGI DECAYING BOTH HARDWOODS AND SOFTWOOD
| Name | Synonyms | Associated rot-type and comments |
| Antrodiella sp. | (Poria undata sensu G. Cunn.) | White pocket rot with small, fleck-like cavities of hardwoods and occasionally softwoods. |
| Bondarzewia berkeleyi (Fr.) Bondartsev &Singer |
(Grifola berkeleyi) (Polyporus berkeleyi) |
White butt rot. |
| Fomes hemitephrus (Berk.) Cooke |
(Fomitopsis hemitephra ) (Heterobasidion hemitrphrum) |
White heart rot with orange zone lines of hardwoods and occasional softwoods (Fig. 18). |
| Inonotus lloydii (Cleland) P.K. Buchanan & Ryvarden |
(Phellinus lloydii) (Fomes lloydii) | Pocket heart rot; "kaikaka" of Podocarpus totara, P. hallii, (Fig. 6). |
| Phellinus senex (Nees & Mont.) Imazeki |
(Fomes senex) | White pocket heart rot of hardwoods and occasionally softwoods. |
| Phellinus wahlbergii (Fr.) D.A. Reid |
(Fomes hamatus) (Phellinus laurencii) (Phellinus zealandicus) |
White pocket heart rot; cavities with brown mycelium; occurrence in softwoods infrequent (Fig. 5, 19). |
| Pholiota adiposa (Batsch) Kummer |
Heart rot in Hoheria angustifolia. | |
| Tyromyces guttulatus sensu G. Cunn. |
In living Ixerba brexioides. |
FUNGI DECAYING HARDWOODS ONLY
| Name | Synonyms | Associated rot-type and comments |
| Auricularia cornea Ehrenb. |
(Auricularia polytricha) (Hirneola polytricha) |
On dead limbs, sometimes descending into the living trunk. |
| Australoporus tasmanicus (Berk.) P.K. Buchanan & Ryvarden |
(Fomes cuneatus) (Fomitopsis tasmanica) (Heterobasidon tasmanicum) |
White heart rot (Fig. 20). |
| Grifola colensoi (Berk.) G. Cunn. |
(Polyporus colensoi) | Brown cubical butt rot mainly on Nothofagus spp. |
| Hymenochaete cervina Berk. & M.A. Curtis |
(Hymenochaete corticolor) | Pocket rot of Nothofagus fusca. |
| Rigidoporus laetus (Cooke) P.K. Buchanan & Ryvarden |
(Coltricia laeta) | White heart rot (Fig. 20). |
FUNGI DECAYING SOFTWOODS ONLY
| "Phaeolus schweinitzii" |
Brown cubical butt rot; known only on Agathis australis (Fig. 3); this unnamed native fungus differs from Northern Hemisphere P. schweinitzii, now introduced into New Zealand.. |

Fig. 18 - Fruiting bodies of Fomes hemitephrus in living kamahi (Weinmannia racemosa).
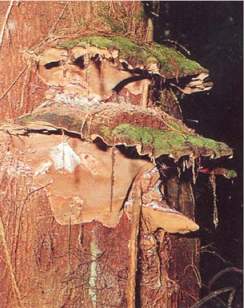
Fig. 19 - Fruiting bodies of Phellinus wahlbergii.
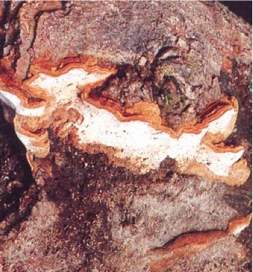
Fig. 20 - Fruiting body of Australoporus tasmanicus.
Bibliography
Butcher, J.A. 1974: A practical guide to fungal damage of timber and wood products. New Zealand Forest Service Information Series No. 65.
Gadgil, P.D. 2005: Fungi on trees and shrubs in New Zealand. Fungi of New Zealand 4. Fungal Diversity Research Series 16: 1-437. Fungal Diversity Press, Hong Kong.
Gilmour, J.W. 1966: The pathology of forest trees in New Zealand. New Zealand Forest Service, Forest Research Institute, Technical Paper No. 48.
Hood, I.A. 1991: Fungi. Chapter 17 (pp. 101-108) in "Botany of Rotorua", comp. B.D. Clarkson, M.C. Smale and C.E. Ecroyd. Forest Research Institute, Rotorua, New Zealand.
Hood, I.A. 1992: An illustrated guide to fungi on wood in New Zealand. Auckland University Press in association with the New Zealand Forest research Institute (424 pp.).
Hood, I.A.; Gardner, J.F. 2009: Fungi decaying stems of fallen tawa (Beilschmiedia tawa) trees in the central North Island of New Zealand. New Zealand Journal of Botany 47: 115-119.
Hood, I.A.; Sandberg, C.J.; Kimberley, M.O. 1989: A decay study of windthrown indigenous trees. New Zealand Journal of Botany 27: 281-297.
Spiers, A.G.; Brewster, D.T. 1997: Evaluation of chemical and biological treatments for control of Chondrostereum purpureum infection of pruning wounds in willows, apples and peaches. New Zealand Journal of Crop and Horticultural Science 25: 19-31.
Compiled: 1986, updated 2009.
This information is intended for general interest only. It is not intended to be a substitute for specific specialist advice on any matter and should not be relied on for that purpose. Scion will not be liable for any direct, indirect, incidental, special, consequential or exemplary damages, loss of profits, or any other intangible losses that result from using the information provided on this site.
(Scion is the trading name of the New Zealand Forest Research Institute Limited.)

 Farm Forestry New Zealand
Farm Forestry New Zealand

