PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Towards a treatment regime for Red Needle Cast
Scion is the leading provider of forest-related knowledge in New Zealand
Formerly known as the Forest Research Institute, Scion has been a leader in research relating to forest health for over 50 years. The Rotorua-based Crown Research Institute continues to provide science that will protect all forests from damage caused by insect pests, pathogens and weeds. The information presented below arises from these research activities.
From Forest Health News 273, July 2017.
Research is underway at Scion to determine how to manage red needle cast (RNC) of Pinus radiata caused by Phytophthora pluvialis. Approaches being considered include breeding for resistance and fungicidal control. A copper-based compound has shown promise as an effective chemical control treatment (FH News 271, March 2017). However, in order to determine when to apply this fungicide, we need to understand the life cycle and behaviour of P. pluvialis.
RNC shows a seasonality with respect to disease expression (Fig. 1). Reports of symptoms tend to start in autumn and increase to a maximum during winter. The disease becomes less apparent during spring and summer as affected needles are shed and new foliage is produced.
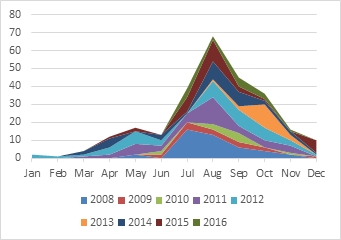
This periodicity is mirrored in studies using pine needle baits floating in traps holding rain water to monitor P. pluvialis inoculum indirectly (FH News 268, July 2016). It was found that in the absence of disease control, infective propagules were available for more than half of the year. In those years in which RNC occurred, inoculum was trapped anywhere between March and December, and even on into January. There were indications that inoculum availability was associated with periods of higher rainfall and cooler temperatures.
Complementary studies have been conducted using potted grafted cuttings of susceptible clones to determine the period in which infection occurs (FH News 271, March 2017). Sets of plants were placed beneath diseased stands, exposing them to available inoculum for two weeks or a month before replacing them with new sets in a succession. This work has confirmed that infection occurs at least between July and October, although it is likely that it may also have been detected outside this period had there been a greater number of plants in the trial. Timing appeared to relate to intervals of greater rainfall recorded by weather stations placed nearby, supporting results from the earlier inoculum monitoring work.

Infection occurred predominantly on exchange plants placed close to diseased stand trees. It was not confirmed on others sited several hundred metres away. This agrees with field observations (Fig. 2), suggesting that general dispersal of P. pluvialis occurs over short distances by means of zoospores in wet or misty conditions. Dothistroma septosporum, the cause of dothistroma needle blight, spreads in a similar way.
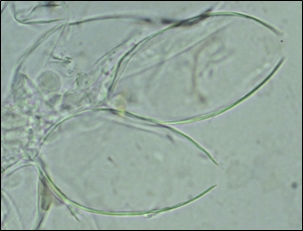
Sporangia of P. pluvialis, some empty of zoospores (Fig. 3), formed on needle lesions on a number of infected exchange plants. This release of zoospores after infection in the same season implies that during outbreak years, red needle cast builds up epidemically in multiple cycles of infection and spore production from autumn through winter until spring.
This scenario, which is consistent with the epidemiology of other Phytophthora spp., suggests that an initial chemical treatment should be applied in autumn as inoculum appears at the start of the season. As the effects of the first application diminish, it may be necessary to apply a second treatment to minimise the rate of new infections. However, more data are needed before a precise recommendation can be made and many questions remain to be answered, not least of which is the manner in which P. pluvialis survives between seasons and outbreak years.
New work planned to address these issues will include two complementary potted plant studies: one to establish a stronger basis for what we understand of the life cycle of the pathogen; and a fungicide trial to determine the best time to apply chemical treatments for optimum control. Although field work can be compromised by the uneven nature of RNC occurrences, it is intended that this research will eventually provide reliable guidelines for disease management.
References
Rolando et al. (2017). Chemical control of two Phytophthora species infecting the canopy of Monterey pine (Pinus radiata). Forest Pathology, 47(3): 1-10.
Williams et al. (2016). Monitoring red needle cast and its relationship with weather factors. Scion report, SIDNEY 57710.
Hood et al. (2017). Infection period of red needle cast on Pinus radiata: fourth phase (2015-2017). Scion report, SIDNEY 58527.
Ian Hood, Nari Williams, and Carol Rolando (Scion)
This information is intended for general interest only. It is not intended to be a substitute for specific specialist advice on any matter and should not be relied on for that purpose. Scion will not be liable for any direct, indirect, incidental, special, consequential or exemplary damages, loss of profits, or any other intangible losses that result from using the information provided on this site.
(Scion is the trading name of the New Zealand Forest Research Institute Limited.)

 Farm Forestry New Zealand
Farm Forestry New Zealand

