PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Pinhole borers, native
Scion is the leading provider of forest-related knowledge in New Zealand
Formerly known as the Forest Research Institute, Scion has been a leader in research relating to forest health for over 50 years. The Rotorua-based Crown Research Institute continues to provide science that will protect all forests from damage caused by insect pests, pathogens and weeds. The information presented below arises from these research activities.
Forest and Timber Insects in New Zealand No. 37: The native pinhole borers
Revised 2009
Limited revision 2001
Based on R.H. Milligan (1979)
Insect: Platypus apicalis White, Treptoplatypus caviceps (Broun)*, Platypus gracilis Broun (Coleoptera: Platypodidae)
* formerly known as Platypus caviceps Broun
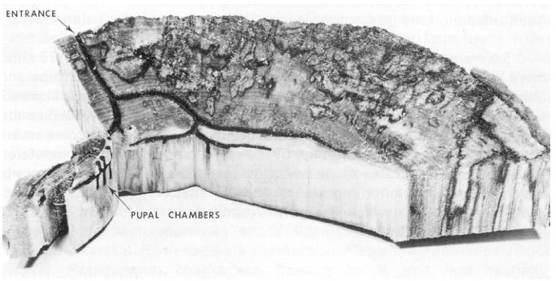
Fig. 1 – Disc cut from Nothofagus fusca (red beech) to show completed nest of Treptoplatypus caviceps.
"Ambrosia" fungi have formed the black coating on the tunnel walls.
Diameter of tunnels 2 mm.
Type of injury
Platypus beetles and their fully grown larvae together make branched systems of cylindrical tunnels which are either 1 mm (Platypus gracilis) or 2 mm (Platypus apicalis and Treptoplatypus caviceps) in diameter throughout. These tunnel systems, or nests, are made in both healthy and weakened living trees, in stumps, in freshly felled logs and larger branches, and occasionally in green sawn timber. The tunnels are almost always across the grain of the wood except for groups of short branches parallel with the grain in which fully grown larvae pupate. The principal branches of each nest tend to lie in one plane. Wood is not ingested to a significant extent; fine strands characteristic of tunnelling by the parent beetles and granular chips excavated by fully grown larvae are ejected from the entrance so that the tunnels are kept free of woody frass. Both adults and larvae feed on yeasts which form a shining coating on the walls of fresh tunnels. In older parts of the nest these yeasts are succeeded by other fungi which form a characteristic blackish coating (Fig. 1). The nests of Platypus apicalis and Treptoplatypus caviceps are essentially limited to the sapwood, but fully grown larvae of Platypus gracilis extend their nests throughout sound moist heartwood. Recently dead, persistently moist wood is the habitat most favourable for the rearing of brood. Adult offspring emerge from the entrance hole made by the parents.
Severe attack on apparently healthy native beech (Nothofagus) 35 cm or more in diameter is usually followed by dieback from the branch tips and, ultimately, death of the tree as a fungus pathogenic to the sapwood spreads from the beetle tunnels in the inner sapwood. Trees of much smaller diameter are also attacked but although some inner sapwood is killed such trees usually survive, and gum flows swamp the beetles long before nests are completed. However, abortive attacks cause timber defects in the living tree which may be of considerable economic importance. Abortive attack is not confined to beech and all tree species in which it is known are included in the following host lists.
Hosts
Platypus apicalis : Brood of this species may be reared in living Nothofagus fusca (red beech), N. menziesii (silver beech), N. truncata (hard beech), N. solandri (black beech and mountain beech), Weinmannia racemosa (kamahi), and Cordyline australis (cabbage tree). However, when Platypus apicalis emerge from living trees the tissues surrounding the nests are dead and so, more precisely, nests may succeed in killed parts of living trees. Death of major branches or dieback of terminals is common when the native beeches are attacked, and similar symptoms of susceptibility to a sapwood pathogen have been associated with attack on living Nothofagus obliqua (a South American species), Castanea sativa (Spanish chestnut), and Brachyglottis (probably B. huntii ). Cabbage trees are not known to be killed.
Abortive attack has been known on living native beeches, Acacia melanoxylon (Tasmanian blackwood), Aristotelia serrata (wineberry), Eucalyptus botryoides, E. delegatensis, E. fastigata, E. gunnii, E. macarthurii, E. nitens, E. obliqua, E. regnans, Populus trichocarpa (western balsam poplar), Quercus robur (pedunculate oak), and Sequoia sempervirens (coast redwood). In at least some of these, attack was concentrated on the more rapidly growing trees in the affected area, and greatest density of attack occurred at those parts of the tree undergoing most rapid increase in diameter. Brood may be reared in felled trees, stumps, logs, and even branches less than 10 cm in diameter of the following (provided they lie in moist situations): the native beeches, kamahi, Agathis australis (kauri), Dacrycarpus dacrydioides (kahikatea), Dacrydium cupressinum (rimu), Pinus muricata (muricata pine), P. nigra (Austrian and Corsican pines), P. ponderosa (ponderosa pine), P. radiata (radiata pine), P. taeda (loblolly pine), Prumnopitys ferruginea (miro), P. taxifolia (matai), Pseudotsuga menziesii (Douglas fir), Acer pseudoplatanus (sycamore), Beilschmiedia tawa (tawa), Elaeocarpus hookerianus (pokaka), and Salix babylonica (weeping willow). Tunnels have also been found in dead Acacia dealbata (silver wattle), Dysoxylon spectabile (kohekohe), Diospyros kaki (persimmon), Picea abies (Norway spruce), Betula pendula (silver birch), Ginkgo biloba (maidenhair tree), Rhus (sumach), and Salix fragilis (crack willow), but it is not known whether brood can be reared in these hosts. Attempted nests occur in dead Eucalyptus delegatensis but no evidence has been found of brood being reared in felled eucalypts. No tunnels have been found in stumps of Chamaecyparis lawsoniana (Lawson cypress) and Cupressus macrocarpa (macrocarpa) adjacent to heavily attacked stumps of radiata pine.
On Chatham Islands, where its principal mainland hosts do not occur, Platypus apicalis has been reported to "attack" Coprosma chathamica, Corynocarpus laevigata (karaka), Melicytus chathamicus, Plagianthus regius subsp. chathamicus (ribbon- wood), Pseudopanax chathamicus, Brachyglottis huntii, and, less frequently, Myrsine chathamica. It is not clear from the report whether "attack" refers to living trees, to those felled or damaged, or to both; some at least, was associated with damage by gales and stock.
Treptoplatypus caviceps: This species is not known to breed in hosts other than living and recently dead red, silver, hard, black, and mountain beeches. Abortive attacks are common in these hosts while alive. Unsuccessful nests have been found in felled Eucalyptus delegatensis, but Treptoplatypus caviceps is not known to attack living eucalypts.
Platypus gracilis: This species breeds in red, silver, hard, black and mountain beeches, Carpodetus serratus (putaputaweta), and kamahi, and brood may emerge from killed parts of these hosts while the trees are still alive. In addition, nests succeed in stumps and large moist logs of beech, kamahi, putaputaweta, various species of Pinus (pine), and Douglas fir. Pines and Douglas fir are recorded as hosts in some exotic conifer forests which include remnant native hosts. In moister parts of dead standing or felled E. delegatensis attempted nests occasionally survive for about a year, but no evidence has been found that eucalypts remain suitable long enough for brood to emerge.
Abortive attack is common in native beeches, in rapidly growing E. delegatensis, E. fastigata, and E. gunnii, and occurs occasionally in Phyllocladus alpinus (mountain toatoa), rimu, and kahikatea.
Platypus gracilis tunnels have been recognised in Metrosideros robusta (rata) and M. umbellata (southern rata), but it is not known whether living trees are attacked or whether brood can emerge from these hosts.
Distribution
Platypus apicalis occurs throughout the North Island and the South Island and on Chatham Islands, but has not yet been found in some of the drier eastern forests (e.g., Balmoral and Eyrewell State forests, Canterbury). Treptoplatypus caviceps occurs wherever beech is present. Platypus gracilis is found throughout the North Island and the South Island especially where there is beech.
It is not known which species exist on Stewart Island and in parts of Westland, where beech is absent.
Economic importance
The three species are of greatest economic importance in beech forests, where they occupy an ecological niche comparable with that of the aggressive bark beetles in coniferous forests of the Northern Hemisphere. They all transmit a fungal pathogen of beech sapwood which, in the event of severe attack, or if light to moderate attack is followed by an abnormally dry season, can kill trees of merchantable size. In addition, sublethal attacks result in “pinhole” damage in heartwood, gum streaks in sapwood, and formation of cores of pathological wood (i.e. irregular cores of killed and stained inner sapwood) (Fig. 2). The presence of pathological wood enables wood-rotting fungi to enter through the stubs of suppressed or broken branches and wounds, so that concealed rot pockets are formed. These are probably the most serious defect encountered in beech sawlogs.

Fig. 2 - Killed and stained inner sapwood (pathological wood) in red beech caused by attack by Treptoplatypus caviceps.
Attack by Platypus apicalis and Platypus gracilis on rapidly growing eucalypts (Fig. 3) used to enrich cutover native forest is likely to greatly reduce the value of timber produced. Meantime it is difficult to quantify such damage because few plantings have reached merchantable size. However, in a minor mill study all pieces sawn from the most rapidly grown of four 30-year-old E. delegatensis bore kino (gum) defects resulting from Platypus attack, while only 2.8% of the pieces from the slowest grown trees were affected (Fig. 4). Kino veins and pockets, which are caused by breakdown of living wood tissue and release of polyphenolic gums or kino, mar timbers intended for clear finishing, spoil veneers, reduce strength of structural timbers, and increase cost of pulping because recovery of chemicals is reduced and greater amounts of bleaching agents are required. Kino veins are not formed in E. gunnii but the Platypus tunnels extend much further into the wood of this species.

Fig. 3 - Platypus damage to lower stem of 10-year-old Eucalyptus nitens .

Fig. 4 - Gum rings (one arrowed) in 30-year-old Eucalyptus delegatensis, caused by annual Platypus attack.
Description
The beetles (Fig. 5) are elongate, cylindrical, and predominantly chestnut-brown or dark brown. The head is broader than it is long, and its front (which may be slightly convex, flat, or distinctly concave) scarcely projects beyond the eyes. The jaws are directed downwards, and the antennae end in a flattened, plate-like club. The prothorax is roughly cylindrical and more than half as long as the elytra (wing cases). On the upper surface of the prothorax there is a narrow median groove and, except in males of Treptoplatypus caviceps, a heart-shaped pattern of fine pits on the rear half. The basal segments of the legs are stout, and the fore tibiae bear conspicuous ridges which serve to anchor the beetle in its tunnel. The terminal segments (tarsi) of the legs are unusually slender and those of the fore legs trail behind as the beetle walks. Specimens cut out of the wood almost invariably have the fore tarsi broken off, and some terminal segments are often missing from the other legs as well.
The elytra are longitudinally ridged, mostly smooth and shining on the upper surfaces except at the rear where there are conspicuous long setae (hairs). In males, this region either bears tooth- like projections at each side of the posterior slope or is strongly tapered, ending in downward directed toothed scrapers. The rear of the females is generally simpler, more rounded, and more densely clothed with long setae. On the underside, there is a much greater distance between the bases of the middle legs and the hind legs than between those of the middle and fore legs. The abdomen is much shorter than the last segment of the thorax, and consists of five visible segments, the terminal one by far the longest and rounded at the rear.
Fig. 5 - Platypus adults. The lines show natural length.

A. Platypus apicalis - width just under 2 mm, less than five times longer than wide; underside between middle and hind legs yellowish.
Left: male, sharp tooth above slope at end of each wing case.
Right: female, steep slope at end of wing cases densely covered with yellow setae.

B. Treptoplatypus caviceps - width just under 2 mm, less than five times longer than wide; underside uniformly brown.
Left: male, wing cases tapered to rear. Right: female, front of head concave.

C. Platypus gracilis - width just under 1 mm, five times longer then wide.
Left: male, two blunt teeth at end of each wing case.
Right: female, rounded swelling above slope at end of each wing case.
The eggs are cream, shining, 0.8-1.0 mm long, 0.3-0.6 mm wide, and rounded at each end. They are found at tunnel ends. The larvae pass through five developmental stages. Youngest larvae are flattened and flounder-like, and bear fleshy finger-like projections of the sides and upper surfaces. They are able to move rapidly along the tunnel walls with an undulating motion. In later stages as the body grows large enough to fill the tunnel it becomes cylindrical and the juvenile projections disappear.
Fully grown larvae (Fig. 6) are creamy white, shining, cylindrical, legless grubs with yellowish heads. The mouthparts are directed downwards, and the jaws are dark brown to black. On each side of the upper surface of the prothorax there is a brown hardened structure which consists of two more or less complete circular ridges linked behind by a transverse ridge, so that the whole resembles a pair of spectacles. Such a structure is lacking in earlier stages of larval development. The end of the abdomen may be smooth or may have a minute pointed spine, or a low hardened ridge, or a blunt tooth.
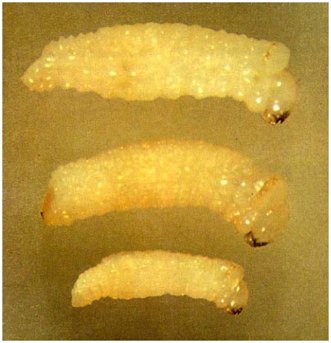
Fig. 6 - Fully grown Platypus larvae. Note the spectacle-like markings on top just behind the head.
Top: Platypus apicalis - width just under 2 mm; small spine at end of abdomen.
Centre: Treptoplatypus caviceps - width just under 2 mm; brown area above blunt tooth or low hardened ridge at end of abdomen.
Bottom: Platypus gracilis - width just under 1 mm; no spine or tooth at end of abdomen.
Larvae at all stages move freely throughout the nest. Pupae are found only in pupal chambers, nearly always with heads directed towards the transverse tunnel from which the chambers arise.
Life histories and habits
Most beetles emerge during the warmer months, though a few may appear on warmer days even in mid-winter. Emergences of Platypus apicalis reach peaks in November and March, and for Treptoplatypus caviceps there are peak periods in November and January. Emergence of Platypus gracilis effectively starts and reaches its peak in January, and then declines through to the end of March.
There are at least three kinds of attractants which guide beetles to their hosts. Males of all three species are attracted to dying or freshly felled trees and stumps by volatile substances given off by the stressed tissues. These males then emit an attractant of their own (probably produced in the hind gut) which causes other males and also females to aggregate at the same place. Rapidly growing eucalypts give off an attractant which causes male Platypus apicalis and Platypus gracilis (but never Treptoplatypus caviceps ) to fly from up to 800 m away to concentrate attack on the most vigorous trees in the stand, and also on those parts (usually the base in young trees) where thickening is greatest. The beetles die before they have penetrated more than a few centimetres. Similar attraction to rapid growth occurs in silver beech, probably accounts for some of the abortive attacks on western balsam poplar and coast redwood, and is very likely of much wider occurrence.
Temporary stress caused by drought or damage to roots may also result in attack on living trees of any species which, if felled or dying, would be utilised by the beetles. Once such stress is relieved, gums or resins soon flood the Platypus tunnels and attractants cease to be emitted. However, if the tree is susceptible to the introduced pathogenic fungus the sapwood is progressively killed, passage of water from soil to crown is restricted, stress is intensified, further attack occurs, and the tree ultimately dies.
Platypus gracilis, unlike the other two species, will establish nests in logs, stumps, and dead standing trees which have been invaded in previous seasons. Males have been seen to start nests in a red beech log felled 20 years previously, and from which the rotted sapwood had fallen away. Nests containing fully grown larvae have also been found in the stump of a large red beech felled 25 years previously.
Males of Platypus gracilis commonly start their tunnels from crevices in the bark or from entrances made earlier by their own or either of the other species. Selection of a concealed site for the nest entrance is not evident in the other two species. The males of all species bore radially through the bark and outer sapwood. A circular groove is cut with the tips of the jaws and strands of wood are then seized at one end and torn away from the tunnel face, the strands being passed backwards beneath the beetle and ultimately ejected at the tunnel entrance. Platypus apicalis and Platypus gracilis accumulate the fibres behind them, compacting them into a cylindrical wad which is steadily forced to the exterior where it protrudes until it breaks and fails. The males do not come right to the entrance while frass is being expelled. Treptoplatypus caviceps lacks an abrupt and toothed posterior suitable for imparting a spiral motion to a compacted wad and, instead, rakes loose bundles of fibres right to the mouth of the tunnel at each sweep. This behavioural difference can be used for the identification of active nests of Platypus apicalis and Treptoplatypus caviceps , the tunnels of which are both 2 mm in diameter, even at times when no frass is being extruded. If a slender straw is gently pushed into the tunnel until the male is touched Treptoplatypus caviceps will push it completely out, so that the end of the insect is briefly visible; Platypus apicalis will push the straw outwards, but not so far that it falls clear of the nest.
Once the male has excavated a sufficient length of tunnel he may be joined by a female. After copulating at the entrance she precedes her mate into the tunnel and takes over the task of extending the nest, the male subsequently ejecting the frass and, at times, excess fungal growth. As the initially radial tunnel continues through the inner sapwood it curves through a right angle and then follows a path close to the boundary between sapwood and heartwood. Should no female arrive, male Platypus apicalis may develop the nest to this stage unaided; some nests initiated in the autumn contain only males in the spring. When no heartwood is present - e.g. when small-diameter living trees are attacked - the tunnel continues radially towards and sometimes through the pith. If either of the pairs should die during the early stages of nest development, the nest fails; if they die at later stages few brood subsequently emerge. If the male has been lost, live larvae may fall from the entrance. So, though it is impossible to observe it in detail, parental tending is of great importance to the brood, and social behaviour patterns are well developed.
When the nest has been sufficiently developed (or perhaps at a given time after copulation), the female lays her first batch of eggs - usually of four to seven - near the end of the tunnel. The presence of the egg batch evidently inhibits further excavations, and a branch in the opposite direction is then started from the curved portion. This second tangential arm is continued until she lays her second batch of eggs, after which the expulsion of fibrous frass ceases. Development of nests to this stage may take 8-10 months.
In the most recently formed parts of the nest a shining coating soon appears on the tunnel walls. This consists of yeasts, which are carried by the beetles (except Treptoplatypus caviceps males) in the minute pits on the rear half of the prothorax. The yeasts bud profusely on the walls of tunnels and develop strands (hyphae) which penetrate a short distance into fresh wood which has not been exploited by other fungi. Larvae have been seen to spread some unknown materials on the walls of such parts of the nest with their mouthparts which include rake-like structures, reminiscent of a simple adhesives spreader. Evidently they cultivate this food resource, and do not simply eat whatever happens to appear. In older parts of the nest the initial coating of yeasts is succeeded by a black fungal growth. While fibrous frass is being ejected much of this growth is abraded, and shortly after adult tunnelling ceases the males scrape excess growth from the walls and eject it as black slimy blobs. Such nest clearings appear periodically from the time adult frass ceases, and are often the last sign of activity after the brood have all emerged. It is unknown whether this black fungal growth plays any part in nutrition. It appears to form a greater part of the flora of Treptoplatypus caviceps nests than in those of the other two species.
In December, January, or February (occasionally earlier or later) after the cessation of adult frass, a different type of woody frass begins to appear. This is granular, and consists of minute, tightly coiled wood chips cut by the fully grown larvae as they begin to extend the parent tunnels or to make new branches. The ends of the tunnels made by the larvae are concave, whereas those made by the parents are flat. Larval frass, at times in great volume, is produced continuously for a year by Platypus apicalis and Platypus gracilis , and for nine months by Treptoplatypus caviceps , before the first adult offspring begin to emerge. Treptoplatypus caviceps larvae contribute least to the final extent of the nest, but they make half the tunnel length which supports an average number of brood; in an average Platypus gracilis nest the larval tunnels extending into heartwood may be several times longer than those made by the parents; Platypus apicalis larval tunnels are less extensive than those of Platypus gracilis but usually more so than those of Treptoplatypus caviceps. These larval extensions of the nest control the numbers that can be reared, because they extend into previously uninvaded wood suitable for the cultivation of more yeasts, and provide more tunnel ends at which egg batches may be deposited.
As well as extending the nest, mature larvae make groups of short blind tunnels (Fig. 1) parallel with the grain of the wood and about 8 mm long, in which they pupate. Groups of pupal chambers usually occur above and below branch tunnels near the junction of the tangential arms and the entrance tunnel. The openings of the upper and lower series are never quite opposite. The number of pupal chambers is not a reliable indication of the number of brood which have emerged from a particular nest; for example, dissection of Treptoplatypus caviceps nests from which 129, 49, and 39 beetles had emerged disclosed only 54, 39, and 32 pupal chambers respectively, so chambers must sometimes be re-used by later-developing brood, especially in unusually productive nests. For larvae which develop from the first egg batches, attainment of the final larval stage is usually followed by a long period of nest extension activity before they pupate; in unfavourable host material, where competing fungi leave no uninvaded wood for yeast cultivation, nest extensions serve no useful purpose and the larvae evidently pupate much sooner.
All three species produce, overall, approximately equal numbers of males and females, though individual nests may produce a marked predominance of either sex. The sexes emerge randomly as far as Platypus apicalis and Treptoplatypus caviceps are concerned, but more than 70% of Platypus gracilis which emerge in the first two weeks of the flight season are males, and the numbers of each sex which have emerged does not become equal until towards the end of February. Most Platypus apicalis and Treptoplatypus caviceps emerge in the flight season two years after nest initiation, though 25% of the total Treptoplatypus caviceps and 40% of Platypus apicalis appear subsequently, mostly in the third but with a few in the fourth season. Such a spread of emergence occurs from nests in relatively stable material. Those in hosts which undergo more rapid biodegradation produce few brood after the first flight season. The most offspring recorded from one nest has been 425 for Platypus apicalis and 129 for Treptoplatypus caviceps , but usually the numbers are much lower. Platypus gracilis begin to emerge 2 years after nests are initiated, but many nests produce no brood (the mean is about two per nest) and there are rarely more than seven, so it seems that the numbers emerging in the first flight season are of the same order as the numbers of eggs in the first batch. This species produces most brood (about 80% of the total) in the third and fourth seasons after nest initiation, but more than half the nests studied in a large-diameter stump and log of red beech continued to produce six years after nest initiation. Though observations were discontinued it seemed clear that some nests could continue to be productive. During this period the maximum number of brood per pair of beetles was 528, and the mean for 40 nests was 115.
In all three species it is usual to find that twice as many nests produce fewer brood than the mean; a high proportion of the total brood appear from rather few, unusually productive nests.
Control
No insect parasites or predators are normally found in Platypus nests. Emerging adults often carry large numbers of mites, but these have not been identified and it is not known whether they are parasitic on adults or their offspring, or are merely scavengers in the nests. Parasitic nematodes have recently been found in the body cavities of all three Platypus species but their significance is not yet known. Control of Platypus species using the entomopathogenic fungi Beauveria bassiana has been investigated, but the methods are not yet resolved. Lenax mirandus Sharp, a slender, sculptured, black beetle belonging to the family Monotomidae, is common in Platypus nests in both the North Island and the South Island. It has been suggested that it is a predator of Platypus but there is no evidence supporting this view. Most probably, it feeds on fungi in the nests without harming its hosts.
Those factors which cause the failure of attempted nests - particularly gum and resin flows when living trees are attacked, and partial drying followed by invasion of the wood by a succession of fungi in dead hosts - are probably the most important in limiting populations.
No control procedures have been attempted in native forests. Traditional selection logging practices, and experimental thinning of pole stands have, indeed, caused populations to reach high levels locally. Although there are no proved control methods, it is possible to suggest forest management practices which would result in least damage to beech grown for the production of special-purpose timbers and veneers, viz:
- Stands should be clearfelled and then regenerated;
- Regeneration should be thinned to final-crop spacing before the wastes are large enough to serve as attractants or the crop trees are susceptible to significant damage;
- Stocking should be such that trees are unlikely to be weakened by competition;
- Tree form should be such that wind throw is least probable- i.e. short and stout, with well-developed root systems;
- Windthrown trees and those broken by snow should be removed as soon as possible. Wastes not removed should be broken down so that they dry or decay rapidly.
Because wide spacing is likely to promote rapid growth rates thus rendering the crop trees attractive, it is important that there are few potential sources of Platypus close to managed stands; logging operations nearby (or any other operation likely to provide a mass attractant) should be avoided.
Use of a cool shaded site for stockpiling logs, though it may reduce damage by sapstain fungi, is likely to promote damage by Platypus, and also by the scolytid beetle Pachycotes peregrinus if the logs are of coniferous species. Until it becomes practicable to control forest insects which breed in stumps, there is always risk of insect damage to logs when stockpiled during the summer. Since freshly attacked logs are most attractive to Platypus, affected logs should be segregated from the pile as soon as they are detected. The application of contact insecticides to prevent damage will rarely be practicable because of difficulty in wetting all surfaces in the pile.
For green sawn timber at sawmills the addition of contact insecticides to the sapstain bath or spray tank should rarely be necessary. If such treatments are required, expert advice should be sought to ensure that appropriate formulations are used otherwise the insecticide may crystalize out in the bath or tank. Any probable source of beetles such as rejected logs or damaged standing trees in the surrounding area should be removed.
Bibliography
Coates, D. 1972: Defects in living silver beech caused by Platypus and Psepholax. New Zealand Forest Service, Forest Research Institute, Forest Entomology Report No. 32 (unpublished).
Emberson, R.M. 1984. Forest and timber insects. In: Scott, R.R. (ed) New Zealand Pest and Beneficial Insects. Lincoln University, Canterbury, New Zealand. P 191-204.
Faulds, W. 1973: Discolouration associated with Platypus wounds in living Nothofagus fusca. New Zealand Journal of Forestry Science 3 : 331-341.
Holloway, W.A. 1973: A study of Platypus caviceps in felled hard beech. New Zealand Forest Service, Forest Research Institute, Forest Entomology Report No. 39 (unpublished).
Glare, T.R., Placet, C., Nelson, T.L. and Reay, S.D. 2002: Potential of Beauveria and Metarhizium as control agents of pinhole borers (Platypus sp.). New Zealand Plant Protection, 55: 73-79.
Kershaw, D.J. 1969: The incidence of Platypus species in living silver beech at Rowallan Forest, Southland. New Zealand Forest Service, Forest Research Institute, Forest Entomology Report No. 24 (unpublished).
Milligan, R.H. 1972: A review of beech forest pathology. New Zealand Journal of Forestry 17: 201-211.
Milligan, R.H. 1974: Insects damaging beech ( Nothofagus) forests. Proceedings of the New Zealand Ecological Society 21 : 32-40.
Milligan, R.H. 1975: Platypus in beech forests. Pp. 48-50 in New Zealand Forest Service, Report of Forest Research Institute for 1974. Government Printer, Wellington.
Milligan, R.H. 1979: Platypus apicalis White, Platypus caviceps Broun, Platypus gracilis Broun (Coleoptera: Platypodidae).The native pinhole borers. New Zealand Forest Service, Forest and Timber Insects in New Zealand No. 37.
Reay, S.D., Hachet, C. Nelson, T.L., Brownbridge, M. and Glare, T.R. 2007: Persistence of conidia and potential efficacy of Beauveria bassiana against pinhole borers in New Zealand southern beech forests. Forest Ecology and Management, 246: 232-239.
Zervos, S. 1980: Bispiculum inaequale n. gen. & sp. (Nematoda: Tetradonematidae) from New Zealand wood-boring beetles (Curculionidae: Platypodinae). New Zealand Journal of Zoology 7: 155-164.
This information is intended for general interest only. It is not intended to be a substitute for specific specialist advice on any matter and should not be relied on for that purpose. Scion will not be liable for any direct, indirect, incidental, special, consequential or exemplary damages, loss of profits, or any other intangible losses that result from using the information provided on this site.
(Scion is the trading name of the New Zealand Forest Research Institute Limited.)



